Second Deloitte report on Covid-19 Supply Chain
May 5, 2021,
by Amanda Creane - Category: Healthcare
Second Deloitte white paper urges stakeholders to develop standards, processes and capabilities so that all citizens across the globe have access to lifesaving therapies.

A new report authored by Deloitte calls for international healthcare organisations to promote global standards, such as the GS1 DataMatrix barcode for product identification across the medical supply chain to ensure citizens around the world have a speedy, equal, and secure access to Covid-19 vaccines.
Access the report
“Now more than ever, leaders in the global health community must develop standards processes, and capabilities to ensure citizens across the globe have access to lifesaving therapies”, states the Deloitte study
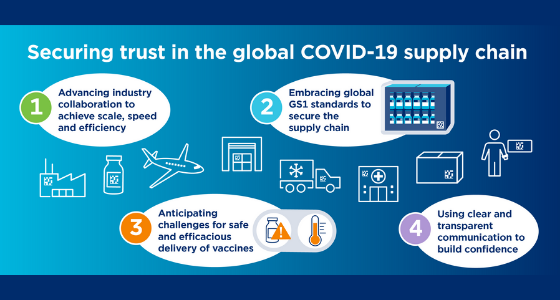
Securing trust in the global supply chain of Covid-19 vaccines, moving from development success to vaccination. Despite the unprecedented level of collaboration by healthcare stakeholders and governments around the globe and the speed in the development of COVID-19 vaccines, today, after more than two years living with the pandemic, there is no reliable, transparent way to verify where vaccines are in many countries, and whether supply is meeting demand. This not only affects confidence in the vaccine supply chain, but it can also exacerbate healthcare inequalities and compromise patient safety.
Adopting a harmonised global standard for identification and traceability would make a difference, explains the Deloitte report. Requiring serialisation of vaccines would allow for more efficient and accurate traceability of vaccine administration and can help fight fake and counterfeit vaccines. In addition, serialisation would make it easier to calculate vaccination rates, identify bottlenecks and recognise where vaccines are being wasted, all issues affecting with particular emphasis the developing countries.
More than 75 countries are requiring or accepting the GS1 DataMatrix, a 2D barcode recommended by healthcare stakeholders that includes critical data such as expiration dates and lot numbers, helping reduce errors and enabling traceability of medical products. The GS1 DataMatrix can be leveraged for “identification and traceability of medical products such as Covid-19 vaccines”, as mentioned in the Deloitte report.
The report, which follows a publication from Deloitte on the same topic released in January 2021, recommends, among other measures, to
“provide vaccines with identification and barcoding on the vial”,
and also commends Ireland’s technology driven approach to vaccine’s data management facilitated by a GS1 standards-based software, similar to those in Ethiopia and Nigeria. Hopefully sooner than later, the Covid-19 pandemic will fade, but the learnings can be crucial to better prepare the world to deal with future health crises.
“We can emerge stronger out of this pandemic if all healthcare stakeholders are committed to enhance collaboration and to transform in concrete solutions the insights gained over the last two years. GS1 stands ready to support these efforts”, Ulrike Kreysa, Senior Vice-President Healthcare at GS1 Global Office.
Access the report
For more information contact:
GS1 Ireland
Siobhain Duggan
Director of Innovation and Healthcare
Tel: +353 86 045 9816
Siobhain.Duggan@gs1ie.org
Tags: traceability, covid19, vaccines, scan4safety