New Deloitte report on Covid-19 Supply Chain
Jan 15, 2021,
by Amanda Creane - Category: Healthcare
New report by Deloitte supports use of GS1 standards for identification, barcoding and traceability across the healthcare supply chain
Deloitte white paper highlights why common standards across healthcare supply chain are essential as the world gears up for the largest deployment of vaccines in history.
Access the report
As governments and businesses around the world face one of the most significant logistical and healthcare related challenges in history, a new report issued by Deloitte points to universal adoption of common standards across the healthcare supply chain as an urgently needed solution to enable fast, efficient and safe distribution and administration of vaccines.
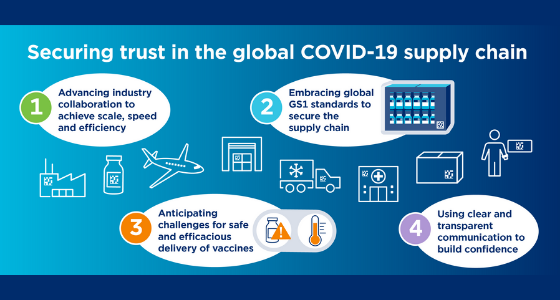
The Deloitte study, “Securing Trust in the Global COVID-19 Supply Chain,” argues that, in addition to industry collaboration and transparent communication, “embracing GS1 standards adds an element of trust at all levels of the supply chain – a trust that ultimately extends to the patients themselves”.
The role of GS1 Standards
GS1 barcodes uniquely and securely identify medical products, including vaccines, from laboratories and clinical trials to point of administration. GS1 standards bring transparency and help to improve supply chain coordination, decreasing the risk of vaccine diversion, date expiration and fake vaccines proliferation.
The Deloitte study calls vaccine identification information (such as product identifier, lot number, and expiration date) “essential for healthcare providers to administer vaccines with confidence”, noting that, “the WHO recommends that all vaccines be identified with this data in a standardised barcode.” GAVI, the Vaccine Alliance, and UNICEF have also required the use of GS1 standards on the secondary packages of vaccines.
Mike Byrne, CEO at GS1 Ireland noted, “an unprecedented level of research, collaboration and investment has brought hope in the form of Covid-19 vaccines. The world now faces an enormous distribution and administration challenge where global identification standards have a critical role to play, and GS1 stands ready to help all stakeholders succeed in this critical operation.”
Greg Reh, Global Life Sciences & Health Care Industry Leader, Deloitte added, “For vaccine developers, health care stakeholders, and society at large, the level of transparency and public trust will determine Covid-19 vaccine acceptance and confidence. The continued adoption of global standards from organisations like GS1 are helping to instill that confidence in the Covid-19 vaccines.”
Today more than 70 countries have healthcare regulations or trading partner requirements for which industry uses GS1 standards. These countries rely on GS1 DataMatrix two-dimensional (2D) barcodes that can encode vaccine identification information to help reduce errors and enable traceability.
As some countries are experiencing tracking difficulties and challenges in linking vaccines to patients at the point of administration, the Deloitte report notes that “it is important to identify and label the vaccines capturing precisely which patient received which vaccine, and when.” Globally unique identification and GS1 barcoding can help with that critical task.
Access the report
For more information contact:
GS1 Ireland
Siobhain Duggan
Director of Innovation and Healthcare
Tel: +353 86 045 9816
Siobhain.Duggan@gs1ie.org
Join the Executive Dialogue on the 28th of January
Tags: traceability, covid19, vaccines, scan4safety