- Home
- Industries
- Resources
- Case Studies
- Healthcare Case Studies
- Traceability standards enable efficient rollout of COVID-19 vaccine.
Loading...
As COVID-19 vaccines became available, Ireland’s Health Service Executive (HSE) needed an efficient and effective way of receiving, administering, tracking and reporting vaccinations across its more than 40 Centralised Vaccination Clinics (CVCs).
Full Video: TrackVax Vaccine Traceability Case Study
Summary Video: TrackVax
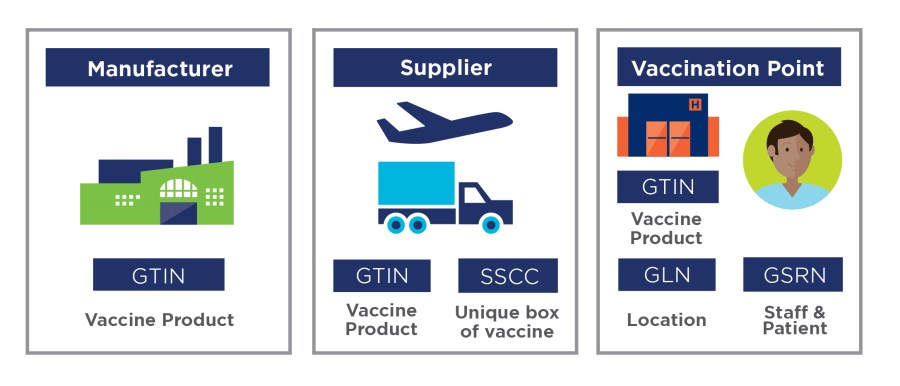
Unique identification is key to enabling full traceability of vaccines
It was important for the HSE’s National Immunisation Office (NIO) that no dose was wasted and that batches of vaccine could be tracked to the point of vaccination.
The feedback on TrackVax from the Senior Management Teams and the High Level Taskforce has been really positive in terms of enabling visibility of vaccine usage and it has been recognised that TrackVax has made a significant contribution to the efficient rollout of the COVID-19 vaccinations across Ireland.
Dr. Lucy Jessop
Director of Public Health - HSE National Immunisation Office
We had approximately 20,000 vaccine deliveries, 4 vaccine types and 20 complicated data fields for each vaccine type. ScanVax standardised everything and meant we could capture records both accurately and quickly. Vaccine data is now seamlessly reportable for the first time in a vaccine programme in Ireland.
Kerry Ryder
ICT General Manager - HSE National Immunisation Office


.png)






