Healthcare systems around the globe are facing challenges that affect the entire supply chain. The sector is concerned primarily with two main issues: patient safety and greater supply chain efficiency and accuracy. The facility to identify things uniquely and accurately is essential, be it a medication, an item of clinical equipment or even a patient. The regulatory landscape continues to evolve globally. New regulations in the EU, the US and elsewhere will have a major direct impact on the healthcare supply chain.
Did you know that between June 9-16, 2015, in just one week, a record 20.7 million fake and illicit medicines, with an estimated value of $81m, were seized, including blood pressure medication, erectile dysfunction pills, cancer medication and nutritional supplements? This was part of Operation Pangea, an international week of action tackling the online sale of counterfeit and illicit medicines and highlighting the dangers of buying medicines online. Coordinated by Interpol, the annual operation brings together customs, health regulators, national police and the private sector from countries around the world.
Ensuring Regulatory Compliance
Product serialisation, compliance with EU, FDA and other international drug pedigree requirements and establishing anti-counterfeiting solutions are key priorities for the pharmaceutical industry. The Falsified Medicines Directive (FMD) took effect on 9th Feb 2019 for all EU member states. The directive which seeks to establish a more secure supply chain for the distribution of prescribed medicines, requires the serialisation of patient packs to include a 2D barcode on all patient packs which are then scanned at the point of dispensing for verification purposes. The legislation also requires tamper evident labelling to enable the authentication of medicines prior to being dispensed to patients.
Achieving Regulatory Value
There is still a lot to do in many areas to achieve compliance however FMD also presents an opportunity to leverage the value of regulatory compliance, particularly given the investment involved. As all prescription packs now have a 2D barcode this barcode can be used to track products internally in the hospital or healthcare provider for the purpose of batch traceability, inventory management, scanning to the patient and more. This legislation along with the regulation for Unique Identification of Medical Devices is driving a harmonised standard for identification in healthcare and industry is working with GS1 to achieve compliance.
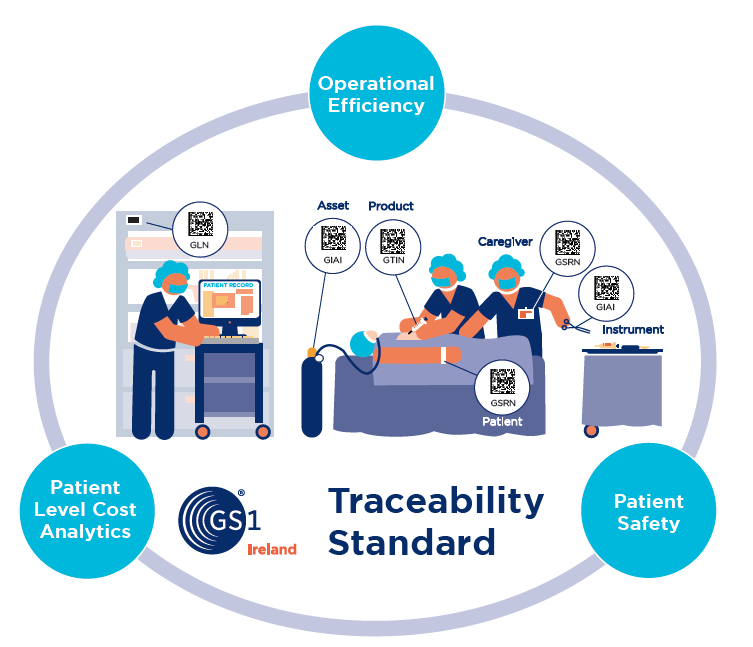
Risks to patient safety occur when there is a mismatch between a patient and the care they receive. Errors can occur at time of diagnosis, treatment or on-going care. When looking at how technology can improve patient safety, we don’t need to look very far. The retail sector has demonstrated the value of adopting GS1 standard barcoding, which has reshaped these industries and created billions of dollars in value.
McKinsey & Co research states: “The potential impact enabled by global standards goes well beyond the use cases that we can identify and quantify today. For example, with global standards in place, payors, regulators and epidemiologists could learn more about the effectiveness of drugs, medical devices and treatments, improving health and yielding savings at the institutional and even national level.” (Strength in unity: The promise of global standards in healthcare, October 2012).
Hospitals and healthcare providers around the world are now working with GS1 to build the foundations for ehealth and electronic capture of data at the point of care. The Scan4Safety programme in the UK is already having a significant impact on patient safety improvements and cost and process efficiencies.
At a National level, the benefits of using 2D barcodes to track and trace Haemophilia medication and surgical instruments and endoscopes are well recognised. HSE Procurement is following these developments and the benefits of unique identification are recognised. “The adoption of GS1 coding standards is viewed as a very important priority to ensure best practices and safety for patients.” Says the HSE’s Head of Procurement John Swords.
The Benefits of adopting GS1 standards in Healthcare
• Improving patient safety
• Lowering costs through increased efficiency
• Reducing medication errors
• Enabling supply chain visibility
• Facilitating effective product recalls
• Tracking pharmaceutical products/medical devices
• Reducing introduction of counterfeit products
• Enhancing inventory management
• Linking critical product data to the patient record
• Supporting regulatory compliance
• Optimising order, invoice, sales reporting, and chargeback/rebate processes
Most recently, Children's Health Ireland at Temple Street have been using the 2D barcodes applied at goods receiving to manage batch traceability for infant feeds within the hospital. Additional benefits of time saving, improved patient safety and improved stock management are also being realised. This is an excellent example of electronic traceability and it has prompted further traceability initiatives within the hospital.
Once the dust has settled on the introduction of the FMD, there is a real opportunity to do more with the 2D barcode on the prescription packs. Please get in touch to learn more.
This article featured in the April 2019 Edition of Hospital Professional News Ireland.
For More information contact:
Siobhain Duggan
Director of Innovation and Healthcare
Tel: 01 208 0660
Email: Healthcare@gs1ie.org
